ReWalk Important Safety Information
INDICATIONS FOR USE
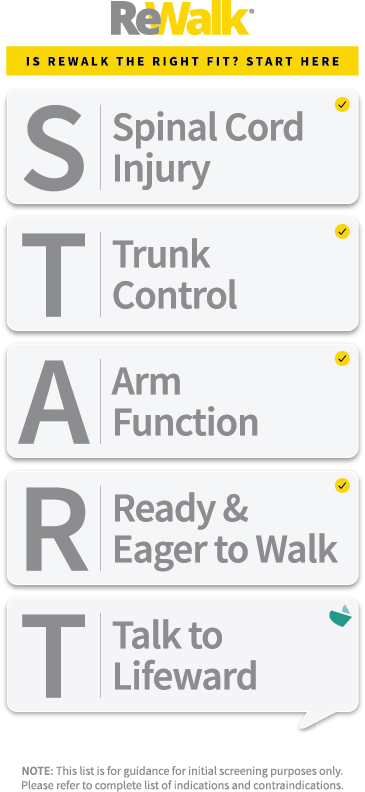
The ReWalk® 7 fits to the lower limbs and part of the upper body and is intended to enable individuals with spinal cord injury at levels T7 to L5 to perform ambulatory functions in home and community settings with supervision of a specially certified companion in accordance with the user assessment and training certification program. The device is also intended to enable individuals with spinal cord injury at levels T4 to T6 to perform ambulatory functions in rehabilitation institutions in accordance with the user assessment and training certification program. The ReWalk 7 is intended for indoor and outdoor use: including standing and walking on level surfaces and mild slopes and ascending and descending stairs and curbs.
INDICATIONS
- Hands and shoulders can support crutches or a walker
- Healthy bone density, meaning sufficient bone density according to the judgement of the prescribing physician after clinical examination of the patient —
- including radiological proof, if needed
- Skeleton does not suffer from any fractures
- In generally good health
- Height is between 160 cm and 190 cm (5′ 3″ – 6′ 3″)
- Weight does not exceed 100 kg (220 lb.)
- At least 18 years old
CONTRAINDICATIONS
- History of severe neurological injuries other than SCI (MS, CP, ALS, TBI, etc.)
- Severe concurrent medical conditions that would interfere with safe device use or walking*
- Uncorrectable Equinovarus foot deformation
- Severe spasticity (Modified Ashworth 4)
- Unable to tolerate standing position in a supported standing device
- Amputations and lower limb prostheses
- Unstable or unhealed fractures of the spine, pelvis and/or lower extremities
- Uncorrectable leg length discrepancy over 2 cm when using additional correction tools
- Heterotopic ossification that impairs joint mobility
- Significant arthritis or contractures that limit lower limb range of motion necessary for adequate walking
- Psychiatric or cognitive disorders that may interfere with proper operation of the device
- Pregnancy
*Please refer to device labeling for full list of contraindicated concurrent medical conditions
WARNINGS & PRECAUTIONS
- The user must be trained to use the ReWalk. Use of the device without training can result in serious injury**
Please refer to device labeling for full list of warnings and precautions
ADVERSE EVENTS
The most common reportable adverse event (AE) that occurs, in less than 4% of ReWalk users, is appendicular fracture
To report suspected adverse events, contact Lifeward at 1-508-251-1154 or FDA at 1-888-INFO-FDA or www.fda.gov/medwatch